Friday, March 25, 2005
Thursday, March 24, 2005
Sunday, March 06, 2005
Just for the record..
All of the previous photos were done only as a proof-of-concept sort of test, and are NOT meant to represent even an attempt at a decent study. I plated some cells tonight which are targeted more at this. I plated from 6 flasks. The first 3, a 1% ITS flask, 1% FBS flask, and 99% OptiMem flask have no ascorbic acid in their media. The second three have been growing with 50ug/mL of ascorbic acid in the flask since I started the flasks 3-3-05. I think having ascorbic acid in the flask should make it so that the cells are fully acclimated to it when they get dumped into the wells. I plated in two 6-well plates; 2 wells per media type so that I have duplicates of everything, and so that there is a control group and an experimental group. I also plated at roughly the same initial cell density (it ranges from 22.75 x 10^4 cells/mL to 38 x 10^4 cells/mL). I personally think that cell density plays a HUGE role in what sort of ECM is produced, and I know from experience that initial plating density is the major factor in eventual confluence, but, as Levar Burton always said.. you don't have to take my word for it. Anyway, with any luck there will be some photos some time late next week.
Saturday, March 05, 2005
Movies
Here are three movies I took with my digital camera, the first shows some of the matrix material moving in the current. The second and third show cells flowing through the matrix. All three movies are encoded with the XVid which is an awsome open source codec. To view these videos you will need to have this codec installed, versions for Windows and Mac can be found here.
New Photos!
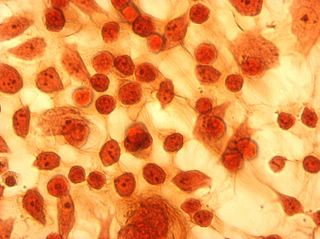
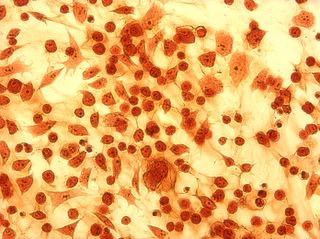
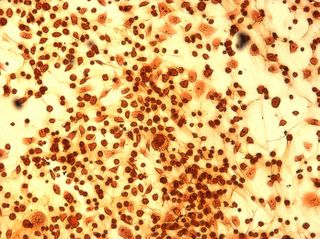
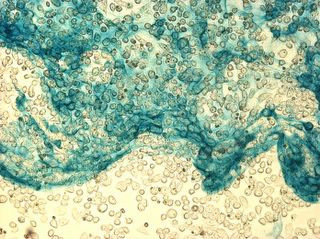
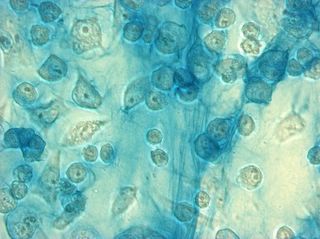
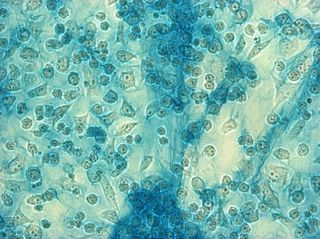
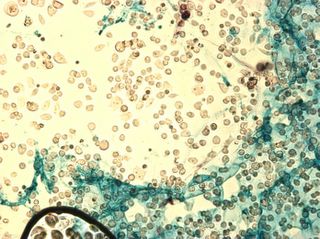
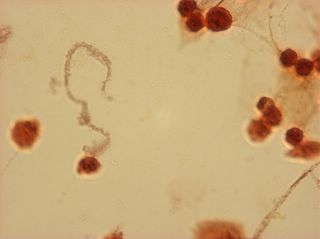
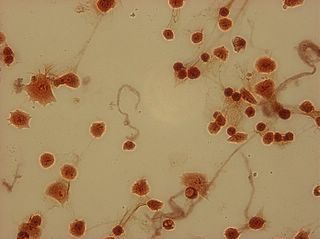
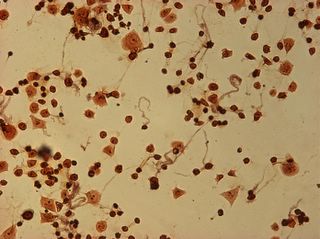
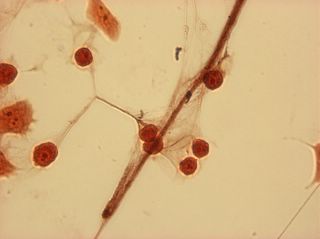
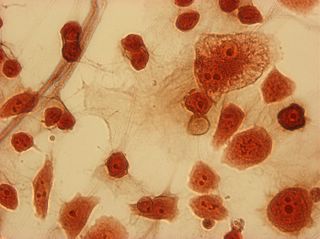
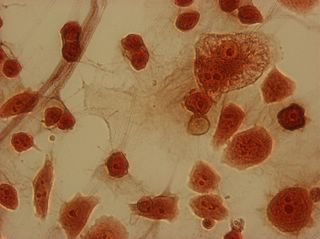
I did my first cell staining of cells treated with ascorbic acid
last night. All of these cells were plated on 3-1-05, and
stained/photographed on the evening for 3-4-05.
The original plating densites were:
1% FBS = 43.5 * 10^4 cells/mL
1% ITS = 15 * 10^4 cells /mL
99% OptM = 35.25 * 10^4 cells /mL
With the high FBS and OptM densities, cells became confluent almost
immediatly. All cells were plated in 50ug/mL ascorbic acid (100ul of
2000ug Asc/1ml mixed with 4mL media). It seems that high initial plating densities are key to plating cells succesfully at all. Cells which are plated at lower densities seem to divide very slowly, and most of them are lost when their media is changed for feeding because only a certain percentage seem be be adherent at any given time.
The orange ones are stained with safranin, and the blue ones are
stained with alcian blue. Cells were fixed for approx 15mins in 1:3
mix of 35% formaldehyde and 1x PBS. (I would have fixed for the normal 10mins but I got locked out of the cell culture room!!!! Luckily things seemed to have turned out fine.)
For the next batch I will try staining with safranin and ruby red,
and for the batch after that, maybe some fluorescent-antibody stain
(although from reading the protocol this looks like a long involved
process).
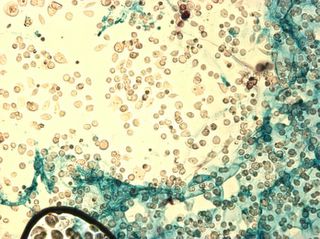
These are cells grown in 99% Optimem, and 1% ITS. Dessication was causing some serious currents in the water, and the cells were actually flowing through channels at quite a rapid rate. I think these channels were formed by different thicknesses of ECM+Cells on the top, and the glass slide on the bottom. I took some small videos which I will try to post as well. These are some time-lapse shots.