Monday, January 31, 2005
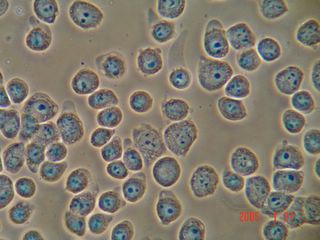
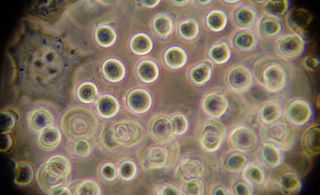
Hematoxylin is supposed to be an acid resistant Nuclei stain. It seemed to just clump and form crystals on this test. Perhaps we needed to mix the stain fresh to get good result.

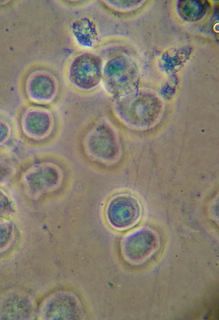
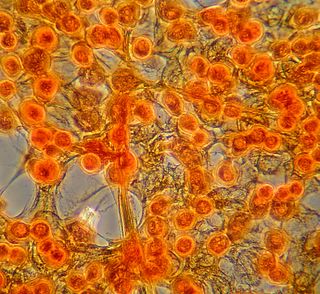
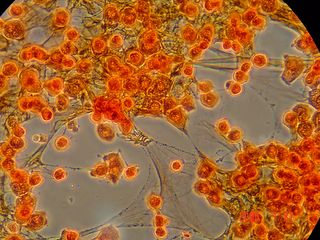
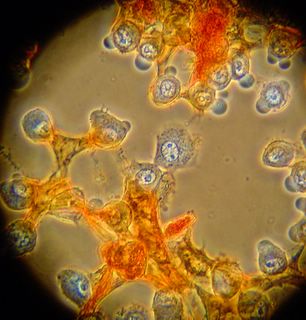
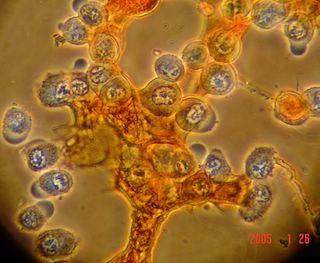
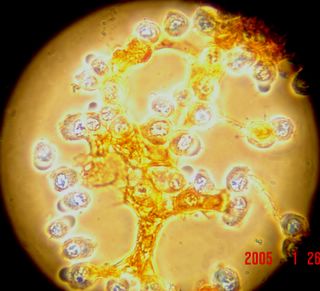
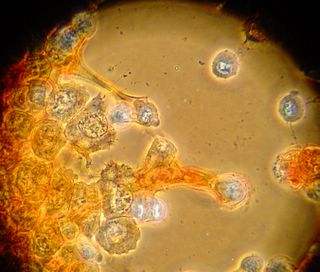
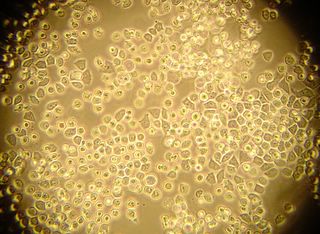
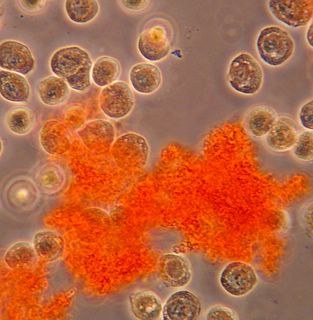
Here is another bradford (aka Coomassie Blue) photo showing what may be tiny fibrils (perhaps collagen?). I didn't even notice these until I look at the photo on a bigger monitor. (Note: all of these images have been sharpened a bit with the Unsharp Mask filter to correct for blurring introduced by the rather archaic photography methodology).

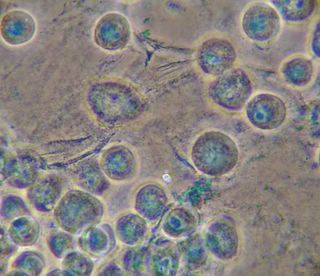
Bradford Reagent which apparently changes it's absorbance spectrum when bound to protein (from 465nm to 595nm) showed a very small amount of protein in the ECM.

Thursday, January 27, 2005
More Staining
Tonight we got a little more advanced in our staining procedure. Here is it:
1. Mix 3 parts PBS fixation buffer with 1 part formaldehyde in a container with a tight fitting lid. (16% formaldehyde is suggested by the protocol we used, but the only stuff we could find was unlabled).
2. Cap tightly and warm the mix in a water bath.
3. Transfer 2ml of the fixation solution above to a watch glass.
4. Remove a cover slip from the 6 well plate with forceps, invert it, and set it on top of the fixation solution.
5. Use a second watch glass to form a small chamber by inverting it and putting it on top of the first.
6. Allow the cells to fix for approximately 10 minutes.
7. Remove the cells from the watch glass and dip twice in deionized water.
8. Place coverslip cell-side-up on a paper towel.
9. Add one or two drops of stain, and allow the stain to sit for 2 minutes.
10. Rinse excess stain with deionized water
11. Place coverslip cell-side-down onto a slide.
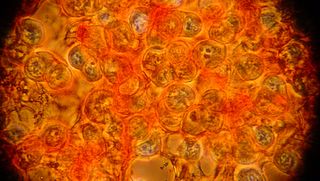
We tried this procedure with Saffron (saffranin), aniline blue, bradford reagent, and hematoxylin. Photos from each type of stain are displayed above (eventually).
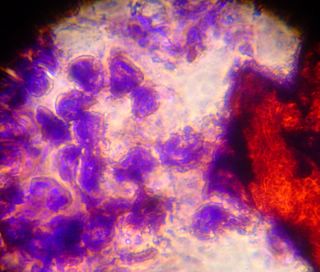
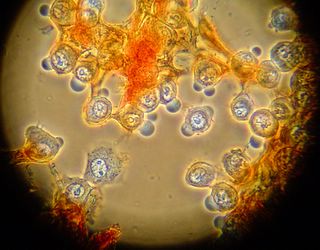
After looking at the saffron stained slide for a while we decided to try adding aniline blue. Unfortunately the cells were unable to withstand the separation of cover slip and slide, and large chunks came free of the cover slip. Here you can see some of the cells stained blue, and some of the connective material stained red. Things were such a mess that it was difficult to see any patterns in these slides. We have begun formulating plans for more gentle staining in the future however.

Wednesday, January 26, 2005
More Staining Information
I found this amazing site which has tons of information about stains, including 15 or 20 which are specifically for collagen. Unfortunately it has few pictures (that I've found so far anyway).
Monday, January 24, 2005
Dang that George Bush..
Well, apparently the stem cells which carry the George Bush Seal of Approval are all contaminated with some non-human surface protein. Of course, good old Pres Bush, in all of his infinite scientific wisdom has said no more stem cell harvests, not matter what the benefits might be. Never mind that stem cell research seems to have a lot of potential for saving lives etc. Oh well, George already has a lot of death on his hands what's a few more I guess?
Sunday, January 23, 2005
Mixing Stains?
So, those are the pictures for aniline blue. I should note that:
I AM NOT CLAIMING TO KNOW FOR SURE THAT ANYTHING I STAINED IS WHAT I THINK IT IS!
Still, the photos are pretty cool looking! (Especially given that I took them with a digital camera held against the eye-piece of the microscope....)
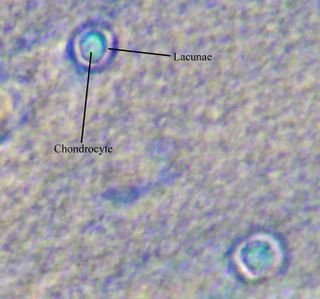
The next step will be to try the other stains individually, and then to try staining a single sample with a couple of different stains. With any luck, I'll have some decent staining procedures worked out about the same time that we have some extra chondrocytes to try them on. Amazingly enough, Tshering was able to find all three stains listed on my first post in a cupboard in Boone! The bottles they are in all look quite a lot older than me, but if they work, it will certainly save ordering and shipping time. Not to mention money!
Wednesday, January 19, 2005
The first:
This is the first of what will probably be many posts. Today I took a sample of bone and meat from a steak in to look at. Dr. Ayers gave Tshering and I some background on the difficulties of staining collagen, and then we did a little bit of messing around with a microscope. As predicted we did not have much luck getting collagen fibers to pick up the stain (Coomassie brilliant blue I believe). We also found that some sort of fungus had made it's home in the stain! Although it looked pretty cool under the microscope, it wasn't exactly what we were hoping for. I took some pictures, some even turned out okay. I will try to post a few eventually!
I just did a little looking around on the web and found this amazing site . Called the "Virtual Histology Lab" it lists dozens of stains and what they stain for. Apparently we need to get our hands on some Saffron, aniline blue, and potassium iodine. Some of these pictures are really amazing (maybe I won't post mine after all.....)